A substance is hard because the smallest particles in its crystal are held together by bonds that, to the maximum possible extent, are high-energy, covalent, and acting in all directions in space.
The Mohs hardness scale
Examples
If shear forces act on an ion crystal, ions of the same charge come into close proximity. These ions repel each other, and the crystal shatters. Substances with ionic bonds are not overly hard. An example is sodium chloride.
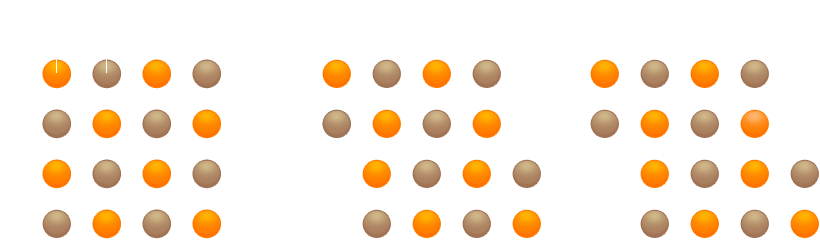
If, in contrast, atoms in a crystal are held together by non-polar bonds, and if shear forces act so as to break these bonds, new bonds will immediately be established between other atoms. The crystal does not shatter. Substances in which all the atoms of a crystal are held together by covalent bonds (atomic bonds) are hard.

The bonds must be covalent
If shear forces act on an ion crystal, ions of the same charge come into close proximity. These ions repel each other, and the crystal shatters. Substances with ionic bonds are not overly hard. An example is sodium chloride.
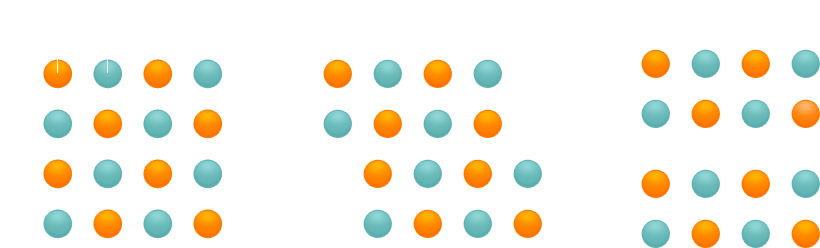
If, in contrast, atoms in a crystal are held together by non-polar bonds, and if shear forces act so as to break these bonds, new bonds will immediately be established between other atoms. The crystal does not shatter. Substances in which all the atoms of a crystal are held together by covalent bonds (atomic bonds) are hard.
The bonds must be pointing in all directions
Only when strong bonds emanate in all directions from the atoms or ions in the crystal is cohesion present in the crystal, and the substance is hard.
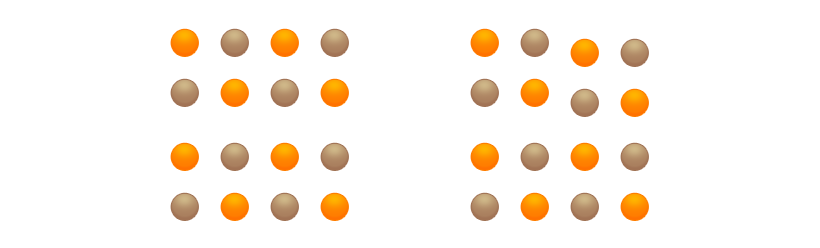
Weak forces that act at right angles to the strong bonds within the layers cannot break the strong bonds. The crystal remains largely unchanged.
It is often the case that strong bonds exist in just 2 directions in space; in the third direction, however, there are only weak bonds. As a result, layers of atoms or ions form, each layer holding together very well in itself, but capable of being easily moved in relation to adjacent layers. Such materials are very soft and can be used as lubricants, for example. One such example is graphite.
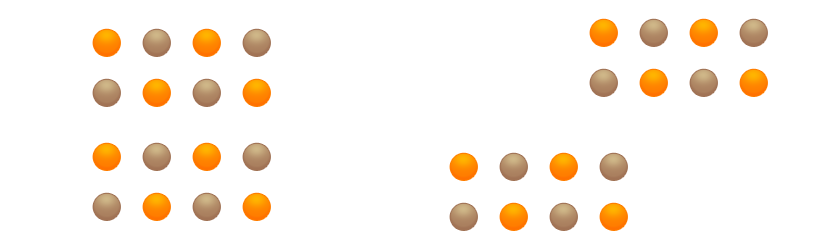
Even quite low shear forces are sufficient to break the weak bonds between the layers. The layers move very easily relative to one another.
The hardness of ionic crystalsIf only ionic bonds are present, the hardness of the material depends on the force with which the ions attract each other. This force is proportional to the charge of the ions and inversely proportional to the square of their separation distance. Ionic crystals, which are composed of small, multiply charged ions, are therefore relatively hard.
How we measure hardness - the Mohs scaleA substance is harder than another one if it can scratch it. The mineralogist Friedrich Mohs (1773 - 1839) drew up a scale in which minerals are arranged in order of increasing hardness.
- Hardness 1: Talc (a very soft mineral)
- Hardness 2: Gypsum (you can just about scratch this with a fingernail)
- Hardness 3: Calcite
- Hardness 4: Fluorite
- Hardness 5: Apatite (you can just about scratch this with a knife)
- Hardness 6: Orthoclase feldspar (K-feldspar)
- Hardness 7: Quartz
- Hardness 8: Topaz
- Hardness 9: Corundum
- Hardness 10: Diamond (the hardest known substance)
To determine the hardness of a substance, one tries to scratch it with the 10 minerals of the Mohs scale in sequence, and then ranks it accordingly.
