A substance is electrically conductive because it contains charged particles that can move freely.
On this pageLearn more about- The conductivity of metals
- The conductivity of graphite
- The conductivity of salts and other ionic compounds
- The unit of conductivity
- Conductivity in numbers
Why do metals conduct current? If atoms have only 1, 2 or 3 valence electrons, neither ionic nor atomic bonds can form between 2 atoms of this element. Ionic bonds cannot be produced, because for this to take place one atom would have to give up its valence electrons (that happens easily with 1 - 3 valence electrons) and the other atom would have to absorb these valence electrons so that, together with its own valence electrons, it can form a noble gas configuration. That is not possible; not enough electrons are present.
Atomic bonds cannot be produced, because for this to take place 2 atoms would have to use their valence electrons jointly in order to populate the valence shell with 8 electrons. Not enough electrons are present to do this, either.
Metallic bonding therefore develops. In this case, all the valence electrons can move freely throughout the whole crystal. They are, so to speak, used by all the atoms jointly. We call this state "electron gas", although it has nothing to do with the gaseous state of matter.
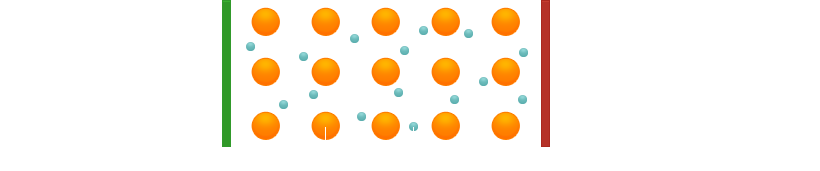
What happens during current conduction in metals? When a voltage is applied, the electrons move slowly through the crystal (and also, of course, through other metal objects such as copper wires).
The most important properties of metallic conductors are
- The conduction of current does not cause any degradation of the conductor
- The conductor has a negative temperature coefficient, i.e. as the temperature increases, the electrical conductivity decreases.
Why do some metals conduct current better than others? This question can be answered with the band model derived from quantum mechanics - not on these pages.
The conductivity of graphiteWhy does graphite conduct current?
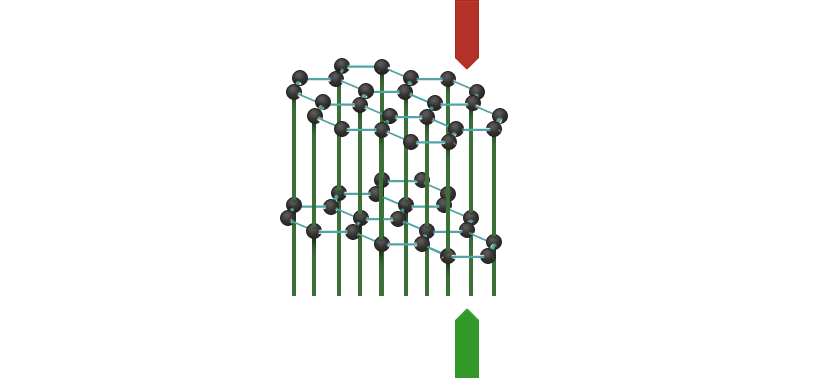
The graphite crystal is built up from layers. Each layer consists of an infinite number of six-membered rings of carbon atoms. Each carbon atom uses three of its valence electrons to form bonds with neighbouring atoms. The other, "fourth" valence electrons form a system of delocalised molecular orbitals, which means that they can move freely throughout the whole layer, and current can be conducted.
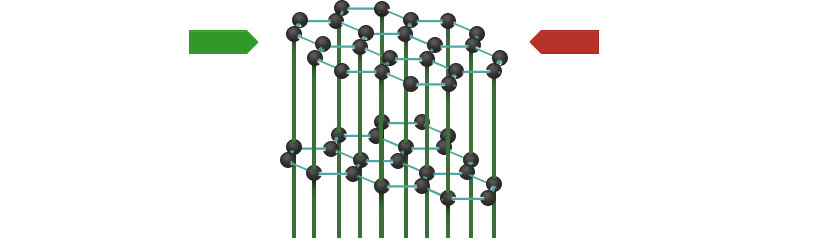
Graphite thus conducts current only within the layers. At right angles to the layers, it is an insulator. We call this phenomenon anisotropy.
In commercially available graphite, a great number of tiny crystals are randomly arranged, so that the layers of individual crystals, which are tilted in relation to one another, make contact. Current is consequently conducted in all directions.
The conductivity of salts and other ionic compoundsWhy do ionic compounds conduct current? Ionic compounds (for example, salts, acids, oxides and hydroxides) are substances that can dissociate into ions. Positively charged ions are called cations, negatively charged ones anions.
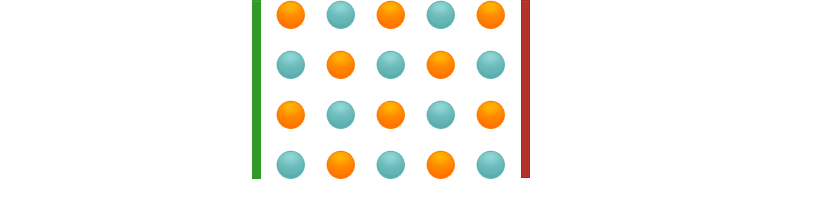
In the solid state, ionic compounds form crystals. In these crystals, the ions cannot move freely, but are located at fixed positions in a crystal lattice. Therefore no current flow can take place.
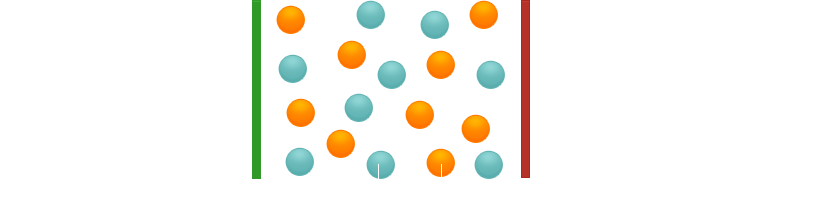
In the liquid (molten) state, the ions can move freely. If a voltage is applied, the cations migrate to the cathode, the anions to the anode and are discharged there. Current flows. The same thing happens in the dissolved state.
What happens during current flow in ionic compounds? When a voltage is applied, the ions migrate: cations to the cathode, anions to the anode.
The most important properties of ionic conductors are
- When current flows, the ionic compound decomposes. Once all the ions have migrated to one of the electrodes, no more freely moveable ions are available (of course!), and no more current flows.
- Ionic conductors have a positive temperature coefficient, i.e. as the temperature increases, the electrical conductivity increases. The reason is the decreasing viscosity of the water. Not until high temperatures does the conductivity decrease again.
Why do metals conduct current better than ionic compounds? The charge carriers in metals are the electrons, in ionic conductors they are the ions. Ions are much larger than electrons, and therefore much less mobile.
Examples of ionic conductors: Sodium chloride
The unit of conductivity (also called conductance) is the Siemens, with the symbol S. For the conversion: 1 S = 1 A/V = 1/Ohm.
The conductivity is therefore the reciprocal of the resistance.
The conductivity L depends on the length l and the cross-sectional area q of the conductor carrying the current, and also, of course, on the material that the conductor is made of. The following applies:

Here, κ (kappa), is the specific conductivity. It is different for each material and depends on the temperature and, in the case of solutions, also on the concentration. The unity of specific conductivity κ is 1/Ohm*cm.
How large is the conductivity?The conductivity of different materials varies greatly. It extends over many orders of magnitude. The graph gives just a rough overview of the order of magnitude in which the conductivities of individual classes of substances are located.
