Graphite
As well as diamond and the fullerenes, graphite is another of the allotropes of carbon. It crystallises hexagonally in the typical lattice layers, whereas diamond crystallises tetrahedrally and fullerenes consist of five- or six-membered rings that have linked up to form balls. The chemical symbol is C.
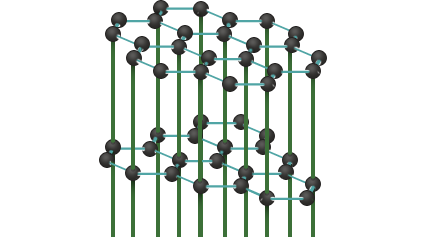
Each carbon atom in a layer is bonded to a carbon atom in the adjacent layer. These bonds are 3.35 Å long, which is more than twice as long as the bonds within a layer (1.421 Å). They are therefore very weak, and graphite can be easily split parallel to the layers.
Occurrence – Extraction – UseSynthetic graphite (artificial graphite)
It is extracted from pitch cokes or petroleum cokes by pyrolysis, with the addition of binders, principally tar and pitch. Pressing operations are used to form it into "green mouldings" under high pressure. In a further step, these mouldings are fired in the absence of air for around 4 weeks at up to 1000 °C to form hard-burnt carbons. In a final step, the actual graphitisation is done by a heat treatment at 2700 °C and above. This process takes a further period of around 10 - 12 days.
The conversion from the amorphous to the graphitised state takes place as a result of the heat that is introduced in the absence of oxygen. The high temperatures produce an extensive orderliness of the carbon-layer planes in the intracrystalline region.
The main production of artificial graphite is as graphite electrodes. Several million tonnes per year are used globally for the production of electrical steel.
Natural graphite
The major deposits are in China, India, Brazil, Mexico and Ukraine. It is extracted there in large quantities from both open-cast and underground mines (around 500,000 tons per year)
Amorphous carbons
These include soots/carbon blacks, charcoals, some activated carbons and uncalcined cokes and anthracites. Their composition is similar to that of graphite. The layers are no longer arranged in parallel, however, but are randomly shifted or twisted relative to one another.
Graphite is a refractory material. It is a lubricant, it is electrically conductive, chemically inert, and it is black. Because of these properties, graphite powders or granules are used in many areas:
crucible production, in lubricants and release agents, brake linings, paints and lacquers, catalytic converters, batteries, membranes and seals, carburising material for foundries
TransformationsAt room temperature and atmospheric pressure, graphite is the thermodynamically stable allotrope of carbon.
With very high pressure (over 100,000 bar), catalysts and high temperature (around 2,400 °C), graphite quickly changes into diamond, which is more thermodynamically stable under these conditions.
Physical properties
| Sublimation point | 3.825 °C |
| Density at 20 °C | 2,26 g/cm3 |
| Hardness on the Mohs scale | 0,5 |
| CAS No. | 7440-44-0 |
THIELMANN GRAPHITE GmbH & Co. KG · Alzeyer Str. 36 · 55459 Grolsheim · Tel.: +49 (0) 6727 / 9312-0 · office@thielmann-graphite.de
